- Visibility 1.2k Views
- Downloads 572 Downloads
- Permissions
- DOI 10.18231/j.ijpi.2021.036
-
CrossMark
- Citation
Peri implantitis- A narrative review
- Author Details:
-
Umar M *
-
Jananni M
-
Saravana Kumar R
-
Pratebha B
Abstract
Dental implant therapy has become the prosthetic standard of care in modern comprehensive dental care. With increase in placement of dental implants, increase in prevalence of peri-implant diseases have also been reported. Two entities are described within the concept of peri-implant diseases; peri-implant mucositis and peri-implantitis. Various etiological factors are responsible for the occurrence of peri-implantitis with bacterial biofilm playing the major role. Appropriate diagnosis and timely management of peri-implant mucositis and peri-implantitis is essential to prevent the implant loss. Variety of treatment modalities are available for management of peri-implantitis which can eliminate the disease progression and enable the restoration of optimal implant function. This narrative review provides insight of the prevalence, etiology, pathogenesis, diagnosis and management of peri-implantitis with emphasis on current evidence.
Introduction
Dental implant therapy has become the prosthetic standard of care in modern comprehensive dental care. With increase in placement of dental implants, increase in prevalence of peri-implant diseases have also been reported. [1] Two entities are described within the concept of peri-implant diseases; peri-implant mucositis and peri-implantitis. Peri-implant mucositis is the reversible inflammation in the soft tissue surrounding implants. Peri-implantitis is an irreversible inflammatory reaction involving the underlying supporting bone. [2]
The bacteria from dental biofilms are considered as the major etiological factor for peri-implant disease. The associated risk factors for peri-implant diseases are alteration in the balance of host-parasite interaction, cigarette smoking, oral hygiene and history of periodontitis. [3] The other possible factors such as genetic traits and implant surface are under investigation. Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans are some of the bacterial species responsible for peri-implant disease. The prosthetic design is also considered as risk factor when the prosthesis obstructs the patient or the dental professional in daily hygiene measures or gaining access to the implant surface. [4]
Peri-implantitis is classified based on severity of the disease as Early, Moderate and Advanced. Different methods have been used to assess peri-implant tissue health and to diagnose these disease entities. [3] The diagnostic methods include evaluation of peri-implant microbiota, peri-implant probing, analyses of peri-implant crevicular fluid or saliva and radiographic evaluation of peri-implant bone loss. The treatment modality includes two types: surgical therapy and non-surgical therapy. Mechanical debridement of the implant surface is the commonest treatment employed for peri-implant diseases.
This article will provide an overview of prevalence, classification, etiology, clinical features, management and maintenance therapy of peri-implantitis with emphasis on current evidence.
Peri-Implantitis
Peri-implantitis is an implant related disease condition which contributes to significant proposition of implant failure. This condition was first described Levignac in 1965. [5] The term peri-implantitis was coined by Mombelli [6] in 1987 who described the disease as equivalent to chronic periodontitis.
According to Frank Schwarz 2017, Peri-implantitis is a pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri- implant mucosa and progressive loss of supporting bone.
Prevalence
Prevalence of peri-implantitis has been reported by various authors and they greatly differ based on number of implants & follow up
|
Author |
Follow up |
Number of implants |
Prevalence (%) |
|
Derks et al (2015) [7] |
- |
- |
42.9% |
|
Aguirre –Zozrano et al (2015) [8] |
5.3 years |
786 |
16% |
|
Daubert et al (2015) [9] |
10.9 years |
225 |
16% |
|
Konstantinidis et al (2015) [10] |
5.5 years |
597 |
6.2% |
|
Derks et al (2016) [11] |
9 years |
2277 |
24.9% |
|
Monje et al (2017) [12] |
3.9 years |
206 |
7.3% |
|
Schwartz et al (2017) [13] |
- |
512 |
7.6% |
|
Tenenbaum et al (2017) [14] |
10.8 years |
108 |
12% |
|
Papaspyridakos et al (2018) [15] |
5.2 years |
457 |
10% |
|
Katafuchi et al (2018) [16] |
10.9 years |
168 |
16.7% |
|
Cosgarea et al (2019) [17] |
9 years |
- |
45% |
|
Francetti et al (2019) [18] |
5 years |
384 |
4.6% |
|
French et al (2019) [19] |
6.7 years |
4591 |
3.6% |
|
Kerbs et al (2019) [20] |
17 to 23 years |
274 |
15% |
C lassification of P eri-Implantitis
Numerous classification systems have been proposed in literature for peri-implantitis. However there is no universally accepted classification so far. The following are the classification systems for peri-implantitis:
Froum and Rosen, 2012classified peri-implantitis based on distinct clinical stages as follows,[21]
Bogaerde et al 2014 proposed classification of bone defects adjacent to dental implants highlighting the defect anatomy in the progression of the regenerative process[22].
Closed defects- It is characterized by the maintenance of intact surrounding bone walls
Open defects- It is the one which lack one or more bone walls.
Ata-Ali et al (2015)classified peri-implantitis based on the clinical status.[23]
Sarmiento et al (2016)classified peri-implantitis based on etiology.[24]
Passi D et al (2016)classified peri-implantitis based on bleeding on probing, probing depth, percentage of bone loss and mobility.[25]
According to Rucha Shah et al (2016), Retrograde Peri-implantitis is classified as,[26]
According to Sarmast et al 2017, retrograde peri-implantitis is classified as,[27]
|
Early |
PD ≥ 4 mm (bleeding and/or suppuration on probing) Bone loss < 25% of the implant length |
|
Moderate |
PD ≥ 6 mm (bleeding and/or suppuration on probing) Bone loss 25% to 50% of the implant length |
|
Advanced |
PD ≥ 8 mm (bleeding and/or suppuration on probing) Bone loss > 50% of the implant length |
|
Staging |
Definition |
|
Stage I |
BoP and/or SUP and bone loss ≤ 3 mm beyond biological bone remodeling |
|
Stage II |
BoP and/or SUP and bone loss > 3 mm and < 5 mm beyond biological bone remodeling |
|
Stage III |
BoP and/or SUP and bone loss ≥5 mm beyond biological bone remodeling |
|
Stage IV |
BoP and/or SUP and bone loss ≥50% of the implant length beyond biological bone remodeling |
|
Origin |
Example |
|
Peri-implantitis induced by pathogenic bacteria/biofilm |
Plaque, calculus, biofilm, previous host susceptibility to periodontitis (previous/active) |
|
Peri-implantitis induced by exogenous irritants |
Residual cement, smoking, impacted food debris |
|
Peri-implantitis induced by iatrogenic factors |
Buccal implant placement, inadequate inter-implant distance, overheating during surgical placement, poorly fitting restorations |
|
Peri-implantitis induced by extrinsic pathology |
Proximal periapical pathology, proximal carcinoma, latent endodontic lesion post extraction |
|
Peri-implantitis induced by absence of keratinized tissue (AKT) |
Absence of attached gingival, lack of keratinized tissue with or without muscle attachment |
|
Stage |
Bleeding on probing |
Probing depth |
Bone loss (%) of implant length |
Mobility |
Proposed treatment and prognosis |
|
Stage 1 |
- |
2-3mm |
10-25% |
No mobility |
No treatment. Oral hygienic instructions; Prognosis is good |
|
Stage 2 |
+ |
4-6mm |
25-50% |
Grade 1 |
Vertical defect <2-4mm |
|
|
|
|
Vertical |
|
Guided bone regeneration, osteoplasty |
|
|
|
|
Horizontal |
|
Horizontal defect< half of implant height Apically positioned flap, Guided bone regeneration, osteoplasty |
|
|
|
|
Combination |
|
Combination defect Bone augmentation & Guided bone regeneration Prognosis is fair |
|
Stage 3 |
+ |
6-8mm |
>50% |
Grade 2 |
Vertical defect 2-4mm |
|
|
|
|
Vertical |
|
Guided bone regeneration Autogenous bone wedge grafting |
|
|
|
|
Horizontal |
|
Horizontal defect> half of implant height Guided bone regeneration and augmentation |
|
|
|
|
Combination |
|
Combination defect Implant removal Questionable prognosis |
|
Stage 4 |
+ |
>8mm |
>50% |
Grade 3 |
Implant removal Poor prognosis |
|
Class I (Mild) |
Extends < 25% of the implant length from implant apex. |
|
Class II (Moderate) |
Extends 25–50% of the implant length from implant apex. |
|
Class III (Advanced) |
>50% of the implant length from implant apex. |
|
Class 1 |
Implant placement resulting in devitalization of adjacent previously vital tooth |
|
Class 2 |
Implant apex infected by persistent periapical lesion on adjacent tooth/implant |
|
Class 3 |
Implant apex placed/angulated labially or lingually outside envelope of bone |
|
Class 4 |
Implant apex lesion developed due to residual infection at placement site |
Classification based on defect morphology
Nishimura et al, 1997 gave another system of classification exists amount of bone loss with shaped of defect associated [28]
Class 1: Slight horizontal bone loss with minimal peri-implant defects
Class 2: Moderate horizontal bone loss with isolated vertical defects
Class 3: Moderate to advanced horizontal bone loss with broad, circular bony defects.
Class 4: Advanced horizontal bone loss with broad, circumferential vertical defects, as well as loss of the oral and/or vestibular bony wall
Schwarz et al 2019 [29] classified peri implant defect depending on the configuration of the bony defect as:
Class I defect – Intraosseous
Class II defect – Supra-alveolar in the crestal implant insertion area.
Spiekermann [30] 1984 characterized peri-implant defect into the type of bone resorption pattern into 5 category.
Class I – Horizontal,
Class II – Hey-shaped
Class III a – Funnel shaped
Class III b – Gap-like
Class IV – Horizontal-circular form
Etiology of peri –implantitis
Though peri-implantitis, like periodontitis is considered a multifactorial disease, two major etiological factors are:
Bacterial infection
Excessive mechanical stress
Bacterial infection- Role of plaque microbiota
Dental plaque or biofilm describes a structure of highly organized microbial community in a well-defined matrix which is adherent to hard surfaces of the oral cavity. A cause- effect relationship between biofilm formation and peri-implant diseases have been demonstrated in humans. [31]
Peri-implantitis present with the mixed and variable microbial pattern, although predominated by gram-negative bacteria.[27] Microbiota associated with failing dental implants is similar to flora commonly associated with periodontally involved teeth. The microorganisms most commonly related to the failure of an implant are rods and motile forms of gram negative anaerobes and spirochetes. These includes: Prevotella intermedia, Porphyromonas gingivalis(PG), Aggregatibacter actinomycetemcomitans(AA), Bacteroides forsythus, Treponema denticola(TD), Prevotella nigrescens, Peptostreptococcus micros and Fusobacterium nucleatum.[32], [33] Some studies have reported that the microbiota of peri-implant cases is different from those periodontal disease. Those studies have detected high numbers of peptostreptococcus and staphylococci species.
The bacterial species of peri-implantitis cases vary between partially and fully edentulous subjects.
Samples from partial edentulous patients have greater gram-negative aerobic species when compared to completely edentulous cases. [34] Periodontal pathogens Aggregatibacter actinomycetemcomitans (AA), Porphyromonas gingivalis (PG) and Treponema denticola (TD) where more commonly reported in partially edentulous cases than completely edentulous cases. [35], [36]
Longitudinal studies have found an increase in a number of colony forming units of bacteria, slight additional increase in pathogenic species and increase in proportion of motile organisms especially spirochetes as severity of disease progresses.
Excessive mechanical stress
It has been postulated that mechanical overloading a contributing factor for peri-implant bone loss and late implant failure. Occlusal overload is influenced by prosthetic design. Implants subjected to occlusal overload results in micro motion at the implant abutment interface and compromises the osseointegration during early healing. [34] The location of implant, the dimension of the implant, proportion of the prosthesis are some of the factors leading to occlusal overload. The occlusal overload also depends on the magnitude, duration, transmitted as lateral or axial stress on to the bone. 34This creates micro fractures of the bone around the implant and initiate peri-implant crestal bone loss which will further propagate in the presence of microbial plaque. [37]
The excess occlusal can be a consequence of placement of implant in poor quality bone, Placement of an implant in poor quality bone, Placement of an implant is an unfavorable position that does not favor ideal load transmission over implant surface, The patient has a pattern of heavy occlusal function associated with para functional habits, Improper prosthetic superstructure that does not fit the implant precisely. [38]
In specific occlusal overload conditions like bruxism, the habit often results in fracture of the suprastructure, but never affects the marginal bone around implants.
Clinical Features
Peri-implantitis is the inflammation of soft tissue around the implant with loss of supporting bone. It corresponds to periodontitis around natural teeth. Early signs of peri-implantitis include increase in GCF production and bleeding on probing. [39] Two important features that differentiates peri-implantitis from peri-implant mucositis are the presence of peri-implant pocket and attachment loss. Additional clinical features are redness of the tissue, swelling of the tissue, suppuration and mucosal enlargement. [40]
Other important features late stage peri-implantitis are implant mobility and pain on function. The classical feature of peri-implantitis is bone loss. Although large variation exist regarding the amount of bone required to define a case. 8th European workshop of periodontology in 2012 reported that > 2mm bone loss from the expected marginal level is considered as case definition. [41]
Guidelines for diagnosing peri-implantitis
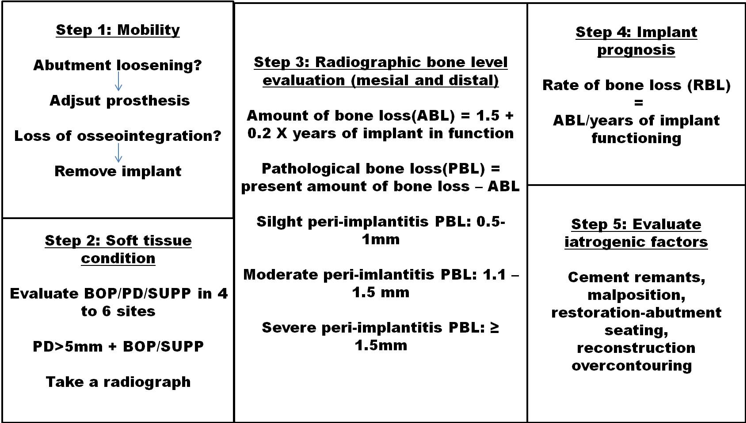
Management
Based upon the clinical and radiographic diagnosis, certain protocols for preventive and interceptive therapeutic measures have been proposed for implants diagnosed with peri implantitis. Lang et al (2004) reported that the system of supportive therapy is cumulative and consists of few steps. The major clinical parameters used are Presence of biofilm, Presence or absence of bleeding on probing (BOP), Presence or absence of suppuration, increased peri-implant Probing depth and evidence and extent of radiographic alveolar bone loss.
Oral implants without plaque and calculus with healthy peri-implant tissue there may be absence of BOP, suppuration, Probing depth should not exceed greater than 3mm to be considered as clinically stable and these sites should not be exposed to therapeutic measures. The indications of appropriate treatment for the management of peri-implantitis lead to the development of treatment protocols.
CIST (Cumulative Interceptive Supportive Therapy) Protocol by Mombelli and Lang(1998) [43]
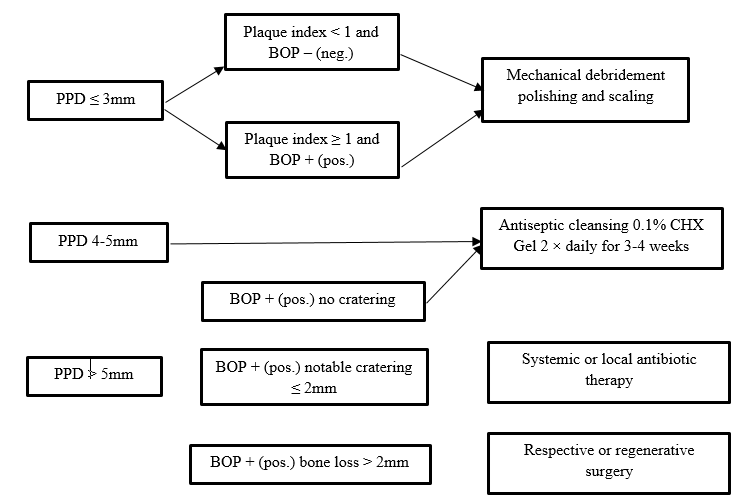
|
Stage |
Result |
Therapy |
|
|
PD<3mm, no plaque or BOP |
No therapy |
|
A |
PD<3 mm, plaque and/or BOP |
Mechanical cleaning, polishing |
|
B |
PD 4-5 mm, no radiographic bone loss |
Mechanical cleaning, polishing, OHI + local antiinfective therapy |
|
C |
PD> 5mm, radiographic bone loss <2 mm |
Mechanical cleaning, polishing, microbiological test, local and systemic anti‑infective therapy |
|
D |
PD > 5 mm, radiographic bone loss >2 mm |
Resective or regenerative surgery |
Maintenance Program
Maintenance therapy after implant placement is of utmost important for effective functioning of implants without any disease. It is classified into two categories.
At home implant care
Professional hygiene care [44]
|
At home implant care |
Professional hygiene care |
|
Brushing |
Scaling and curettage |
|
Soft manual tooth brush |
Plastic instruments |
|
Motorized tooth brush |
Plastic instruments reinforced with graphites |
|
Sonic tooth brush |
Gold-plated curette |
|
End tufted brush |
Ultrasonic or sonic scalers covered with plastic sleeve |
|
Interproximal cleaning/circumferential cleaning |
Polishing |
|
Floss, foam tips, Disposable wooden picks, Interproximal cleaners |
Rubber cup with non-abrasive polishing paste E.g.: aluminum oxide, low abrasive dentifrice |
|
Locally applied therapeutics |
Locally applied therapeutics |
|
Chlorhexidine digluconate |
Arestin, Periochip, Atridox |
|
Water irrigation |
Subgingival irrigation |
|
Hydro floss |
Antiseptic agents such as peroxide, chlorhexidine using a plastic irrigation tip. |
Summary and Conclusion
Use of dental implants for replacement of missing teeth has become the norm in oral rehabilitation in recent days. Though implants have shown effective and predictable results, increase in use of dental implants has also been associated with occurrence of implant failures of which the most common cause is peri-implantitis. Peri-implantitis is progressive destructive chronic diseases of bacterial or mechanical origin that affects hard and soft tissues surrounding the implant.
The purpose of this paper is to review and report the available evidence for various aspect of peri-implantitis; prevalence, etiology, pathogenesis, management and maintenance.
On analyzing the literature for prevalence of peri-implantitis, the prevalence of peri-implantitis at implant level ranged between 6.5% -47% and prevalence at patient level ranged between 18.8% - 47%.
Looking at etiology, dental plaque and associated micro-organisms has been universally identified as the primary etiologic agents. The second factor identified as etiology is the mechanical factors. Apart from these, a number of risk factors have been associated with peri-implant diseases like local factors (parafunctional habit, local anatomic factors, etc.), systemic factors (age, genetics, medical conditions, etc.), behavioral factors (oral hygiene maintenance, patient compliance).
On analyzing the effects of systemic diseases on periodontitis diabetes was the most commonly investigated risk factor. Patients with uncontrolled diabetes were found to be at a higher risk peri-implantitis when compared to controlled diabetes and healthy sites.
On analyzing the evidence for microbiologic profile of peri-implantitis, conflicting results have been reported, but the following micro-organisms were more prevalent Porphyromonas gingivalis (PG), Prevotella intermedia, Treponema denticola (TD), Tannerella forsythia, staphylococcus species, Epstein-Barr virus and human cytomegalo virus 2.
On analyzing the diagnostic evidence of peri-implantitis numerous studies have reported the effects of peri-implantitis on inflammatory cytokines. According to evidence IL1β and TNFα were the most studied pro-inflammatory cytokines. The levels of theses cytokines were found to be significantly higher in peri-implantitis sites when compared to healthy sites. Other cytokines like IL-7, IL-6 and IL-10 have also been investigated but strong association have not been reported.
Looking into treatment of peri-implantitis both non-surgical and surgical interventions have been analyzed. Non-surgical therapy included alteration of implant surface, soft tissue debridement and adjunctive use of anti-microbial, lasers and host modulatory agents. Non-surgical therapy is effective only in eliminating the local etiologic factors and might not the effective in osseous defects. Surgical interventions include reflection of flap, debridement and use of various bone grafts material and membranes. Surgical approach with placement of bone grafts in combination with GTR membranes have been proven to be most effective treatment option with long term predictable result.
With increase in awareness and demand for replacement of missing teeth with dental implants, peri-implant diseases are also increased. Therefore, understanding the etiology, pathogenesis and strategies for treatment for the same should be given equal importance.
Source of Funding
This work not supported in any foundation.
Conflict of Interest
The authors declare no potential conflicts of interest concerning the authorship and publication of this article.
References
- Shah R, Raison T, Kumar ABT, Dhoom SM. A radiographic classification of retrograde periimplantitis. J Contemp Dent Pract. 2016;17(4):313-21. [Google Scholar]
- Sarmast ND, Wang HH, Sajadi AS, Angelov N, Dorn SO. Classification and clinical management of retrograde peri-implantitis associated with apical periodontitis: a proposed classification system and case report. J Endod. 2017;43(11):1921-4. [Google Scholar]
- Passi D, Singh M, Dutta SR, Sharma S, Atri M, Ahlawat J. Newer proposed classification of periimplant defects: A critical update. J Oral Biol Craniofac Res. 2017;7(1):58-61. [Google Scholar]
- Fiorellini JP. A classification system for peri-implant diseases and conditions. Int J Periodontics Restorative Dent. 2016;36(5):699-705. [Google Scholar]
- Ata-Ali J, Ata-Ali F, Bagan L. A classification proposal for peri-implant mucositis and peri-implantitis: a critical update. Open Dent J. 2015;9:393-5. [Google Scholar] [Crossref]
- Bogaerde LV. A proposal for the classification of bony defects adjacent to dental implants. Int J Periodontics Restor Dent. 2004;24:264-71. [Google Scholar]
- Stuart F, Paul F. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32:533-40. [Google Scholar]
- Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontology. 2000;66(1):255-73. [Google Scholar]
- Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002. [Google Scholar]
- Lindhe J, Meyle J. Peri-implant diseases: consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8):282-5. [Google Scholar]
- Karring ES, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri-implantitis by the Vector system. Clin Oral Implants Res. 2005;16:288-93. [Google Scholar]
- Mombelli A, Oosten MAV, Jr ES, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2(4):145-51. [Google Scholar]
- Costa GC, Aras M, Chitre V. Failure in dental implants. Adv Dentl Med Sci. 2014;2:68-81. [Google Scholar]
- French D, Grandin HM, Ofec R. Retrospective cohort study of 4,591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J Periodontol. 2019;90(7):691-700. [Google Scholar]
- Krebs M, Kesar N, Begić A, Krockow NV, Nentwig GH, Weigl P. Incidence and prevalence of peri-implantitis and peri-implant mucositis 17 to 23 (18.9) years postimplant placement. Clin Implant Dent Related Res. 2019;21(6):1116-23. [Google Scholar]
- Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: prevalence of Peri-implantitis. J Dent Res. 2016;95(1):43-9. [Google Scholar]
- Konstantinidis IK, Kotsakis GA, Gerdes S, Walter MH. Cross-sectional study on the prevalence and risk indicators of peri-implant diseases. Eur J Oral Implantol. 2015;8(1):75-88. [Google Scholar]
- Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86(3):337-47. [Google Scholar]
- Aguirre-Zorzano LA, Estefanía-Fresco R, Telletxea O, Bravo M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin Oral Implants Res. 2015;26(11):1338-44. [Google Scholar]
- Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95(1):43-9. [Google Scholar]
- Tenenbaum H, Bogen O, Séverac F, Elkaim R, Davideau JL, Huck O. Long-term prospective cohort study on dental implants: clinical and microbiological parameters. Clin Oral Implants Res. 2017;28(1):86-94. [Google Scholar]
- Monje A, Wang HL, Nart J. Association of preventive maintenance therapy compliance and peri-implant diseases: a cross-sectional study. J Periodontol. 2017;88(10):1030-41. [Google Scholar]
- Papaspyridakos P, Bordin TB, Kim YJ, Defuria C, Pagni SE, Chochlidakis K. Implant survival rates and biologic complications with implant-supported fixed complete dental prostheses: A retrospective study with up to 12-year follow-up. Clin Oral Implants Res. 2018;29(8):881-93. [Google Scholar]
- Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri-implantitis: A cross-sectional radiographic analysis. J Clin Periodontol. 2018;45(2):225-32. [Google Scholar]
- Cosgarea R, Sculean A, Shibli JA, Salvi GE. Prevalence of peri-implant diseases-a critical review on the current evidence. Braz Oral Res. 2019;33(1). [Google Scholar] [Crossref]
- Francetti L, Cavalli N, Taschieri S, Corbella S. Ten years follow-up retrospective study on implant survival rates and prevalence of peri-implantitis in implant-supported full-arch rehabilitations. Clin Oral Implants Res. 2019;30(3):252-60. [Google Scholar]
- Schwarz F, Becker K, Sahm N, Horstkemper T, Rousi K, Becker J. The prevalence of peri-implant diseases for two-piece implants with an internal tube-in-tube connection: a cross-sectional analysis of 512 implants. Clin Oral Implants Res. 2017;28(1):24-32. [Google Scholar]
- Nishimura K, Itoh T, Takaki K, Hosokawa R, Natio T, Yokota M. Periodontal parameters of osseointegrated dental implants. A 4-year controlled follow-up study. Clin Oral Implants Res. 1997;8:272-8. [Google Scholar]
- Monje A, Pons R, Insua A, Nart J, Wang HL, Schwarz F. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Related Res. 2019;21(4):635-43. [Google Scholar]
- Kühl S, Zürcher S, Zitzmann NU, Filippi A, Payer M, Dagassan-Berndt D. Detection of peri-implant bone defects with different radiographic techniques-a human cadaver study. Clin Oral Implants Res. 2016;27(5):529-34. [Google Scholar]
- Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-9. [Google Scholar]
- Charalampakis G, Leonhardt �, Rabe P, Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: A retrospective multicentre study. Clin Oral Implants Res. 2012;23(9):1045-54. [Google Scholar]
- Fürst MM, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res. 2007;18:501-8. [Google Scholar]
- Mombelli A, Lang NP. Microbial aspects of implant dentistry 2000. Periodontology. 1994;4:74-80. [Google Scholar]
- Kocar M, Seme K, Hren NI. Characterization of the normal bacterial flora in peri-implant sulci of partially and completely edentulous patients. Int J Oral Maxillofac Implants. 2010;25(4):690-8. [Google Scholar]
- Karbach J, Callaway A, Kwon YD, Hoedt B, BA. Comparison of five parameters as risk factors for peri-mucositis. Int J Oral Maxillofac Implants. 2009;24(3):491-7. [Google Scholar]
- Lindhe J, Meyle J. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8):282-7. [Google Scholar]
- Soldatos N, Romanos GE, Michaiel M, Sajadi A, Angelov N, Weltman R. Management of retrograde peri-implantitis using an air-abrasive device, Er,Cr:YSGG laser, and guided bone regeneration. Case Rep Dent. 2018. [Google Scholar]
- Heitz-Mayfield LJ. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008;35:292-304. [Google Scholar] [Crossref]
- Tecco S, Grusovin MG, Sciara S, Bova F, Pantaleo G, Capparé P. The association between three attitude-related indexes of oral hygiene and secondary implant failures: A retrospective longitudinal study. Int J Dent Hyg. 2018;16:372-9. [Google Scholar] [Crossref]
- Sanz M, Chapple IL. Working Group 4 of the VIII European Workshop on Periodontology Clinical research on peri-implant diseases: Consensus report of Working Group 4. J Clin Periodontol. 2012;39(12):202-6. [Google Scholar] [Crossref]
- Ramanauskaite A, Juodzbalys G. Diagnostic Principles of Peri-Implantitis: a Systematic Review and Guidelines for Peri-Implantitis Diagnosis Proposal. J Oral Maxillofac Res. 2009;7(3). [Google Scholar] [Crossref]
- Mombelli A, Lang NP. The diagnosis and treatment of peri‑implantitis. Periodontol. 1998;17:63-8209. [Google Scholar]
- Schenk G, Flemmig TF, Betz T, Reuther J, Klaiber B. Some clinical and radiographical features of submerged and non-submerged titanium implants. A 5-year follow-up study. Clin Oral Implants Res. 1997;8(5):427-33. [Google Scholar]
How to Cite This Article
Vancouver
M U, M J, R SK, B P. Peri implantitis- A narrative review [Internet]. IP Int J Periodontol Implantol. 2021 [cited 2025 Oct 30];6(4):204-211. Available from: https://doi.org/10.18231/j.ijpi.2021.036
APA
M, U., M, J., R, S. K., B, P. (2021). Peri implantitis- A narrative review. IP Int J Periodontol Implantol, 6(4), 204-211. https://doi.org/10.18231/j.ijpi.2021.036
MLA
M, Umar, M, Jananni, R, Saravana Kumar, B, Pratebha. "Peri implantitis- A narrative review." IP Int J Periodontol Implantol, vol. 6, no. 4, 2021, pp. 204-211. https://doi.org/10.18231/j.ijpi.2021.036
Chicago
M, U., M, J., R, S. K., B, P.. "Peri implantitis- A narrative review." IP Int J Periodontol Implantol 6, no. 4 (2021): 204-211. https://doi.org/10.18231/j.ijpi.2021.036
