Introduction
Periodontal disease is a chronic multifactorial inflammatory disease caused primarily by dental plaque microorganisms which on progression leads to alveolar bone destruction. Type II diabetes mellitus is a major systemic problem that occurs in middle age people. Periodontal disease is the 6th most complication of Type II diabetes mellitus patients.1 Vitamin D plays a key role in maintaining the integrity of periodontium and its active form inhibits cytokine production and inflammatory cell proliferation.2 In Type II Diabetes mellitus patients inflammatory response will be more to outer stimuli and destruction of the tissue occurs spontaneously. Patients with both periodontal disease and Type II diabetes mellitus were more prone to alveolar bone destruction. By estimating the levels of vitamin D in these patients may give an association of destruction of bone and levels of vitamin D in the blood thus the patients can be kept aware of this association. Hence, the aim of the present study is to assess the serum levels of 25-hydroxyvitamin D in CGP with Type II diabetes mellitus patients in comparison with simple gingivitis patients.
Materials and Methods
Fifty patients with established periodontal disease of score 2.0 to 4.9 in Russel’s periodontal index and type II Diabetes mellitus (CGP with Type II DM) were taken as Group I and Fifty patients with simple gingivitis with periodontal score of 0.3 to 0.9 in Russels periodontal index were recruited as Group II. The present study was done at the Department of Periodontology in G. Pulla Reddy Dental College and Hospital, Kurnool, Andhra Pradesh from the time period of January 2019 to January 2020. Informed written consent has been given to all the patients regarding their participation in the study. All the Patients are of age from 35 to 55 years old. Group, I patients was recruited by recording the probing pocket depths with >/= 5mm with Type II DM patients were included in the test group. Simple gingivitis patients were recruited with probing pocket depth </=3mm without any systemic disease was included for the Control group. All the scores were recorded using William’s probe by measuring the pocket depth from Gingival margin (standardization) to point of resistance apically by a single examiner. Chronic smokers, pregnant ladies, lactating mothers were excluded. The clinical parameters evaluated were Oral Hygiene Index-Simplified (OHI-S), Russell’s periodontal index, serum levels of 25 (OH)D. Radiographically, the presence of infra bony defects was assessed.
Assessment of 25(OH)D
All the patients were advised to take a blood report of 25 (OH)D serum levels immediately after oral prophylaxis was done.
Procedure
Venous blood was drawn from the antecubital vein into serum gel tubes and sera were isolated by centrifugation (10 min at 3220 rpm at 4˚C). Serum levels of 25(OH)D were determined using an enzyme immunoassay) EIASON 25-OH-Vitamin D® test kit. The assessment has been carried out according to the instructions by the manufacturer.
Results
Two patients with established periodontal disease and one patient with simple gingivitis were not reported with the blood test and for further follow-up. All the clinical parameters recorded showed a significant difference between both groups. OHI-S index and Russel’s periodontal index evaluation were interpreted with poor oral hygiene and established periodontal disease in Group I patients whereas with simple gingivitis in Group II patients. The number of intrabony defects present was 2+/-1.1 in Group I patients per two quadrants. In Group II patients there were no recorded intra bony defects (Table 1).
Table 1
Demographic data with clinical parameters between two groups
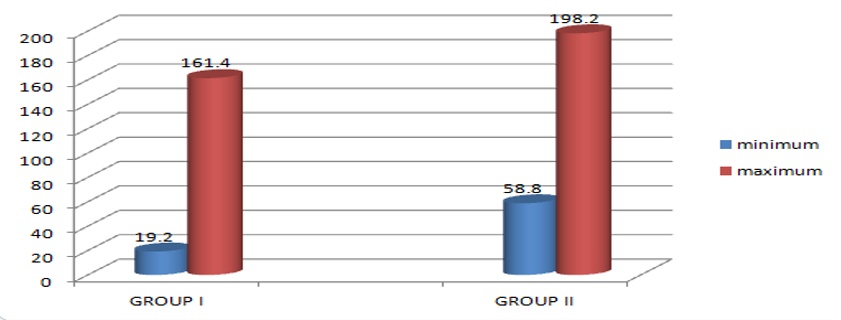
The 25 (OH) D levels were evaluated between the two groups taking the median values of the patient serum levels. For Group I patients the median value was 47.6nmol/l (<50nmol/l is considered normal) with a maximum value of 161.4nmol/l and a minimum value of 19.2nmol/l. In Group II patients the median value was 86.4nmol/l (50 to 100 nmol/l considering sufficient) with a maximum value of 198.2nmol/l and minimum value 58.8nmol/l. the values provided a significant decrease in the levels of 25 (OH) D in patients with CGP and Type II DM which may result in more destructive bone resorption (Figure 1). Though the values vary individually with a wide range the median of group I patients showed significant deficiency in vitamin D levels. Along with this, the overall insufficiency in all the patients can be supported that may be due to the women of age early menopause and menopause were recruited in the group.
Figure 1
Graph showing the assessment of 25(OH)D serum values with maximum and minimum levels in both the groups:
Statistical analysis
Statistical analysis was performed using SPSS Statistical package for social sciences software. Student ‘t’ test was done taking median values to determine the characteristic differences between the two groups in the levels of 25(OH)D, and a p-value of ≤ 0.05 was taken as significant.
Discussion
According to the estimation of the international diabetes federation, the number of people with Type II diabetes mellitus were nearly 285 million, or 7% of the world’s population. This may exceed 435 million by 2030.3 People with diabetes was more prone and are at high risk of inflammatory diseases. Periodontal disease which is a multifactorial inflammatory disease has a strong association with diabetes and had proven as a complication. The most concerning stage of periodontal disease are the stage of bone resorption which is mediated by inflammatory mediators. In patients with diabetes and periodontal disease were highly in danger of early bone resorption and further tooth loss. Vitamin D deficiency is regarded as an epidemic nowadays because 1 billion people across the world were deficient in vitamin D.
Therefore in the present study, the levels of Vitamin D3 were assessed in these patients taking the normal values as a watershed which may further help to know the level of bone resorption undergoing in the body so that proper precautions and medication can be advised to reduce the ongoing destruction. Bone mineral density and bone turnover were always related to the status of Vitamin D. Many previous studies have demonstrated that vitamin D deficiency may place subjects at risk not only by lowering the mineral density but also by putting the subjects more prone to infectious and chronic inflammatory diseases.4 According to Adams and Hewison (2010), calcium homeostasis is the main function of Vitamin D but with a significant role also in maintaining immunity, functioning of the Cardiovascular system, controlling blood sugar levels, etc.5 An alternative hypothesis for low serum 25(OH)D levels suggests that low vitamin D could also be the result of a chronic inflammatory process caused by persistent infection.6 In a study by Markus Laky et al, it was concluded that patients with periodontal disease presented a significantly higher proportion of deficient 25(OH)D levels than their normal counterparts. This was as significant as with the results of the present study.7 A study was done by Holick, (2007)8 states that diagnosis of vitamin D deficiency is made through serum analysis of 25(OH)D levels, the normal range of serum 25(OH) D levels is 20-74 ng/mL. According to Malabanan et al., 1998; Bischoff-Ferrari et al., 2006 there is no absolute threshold for deficiency status is universally accepted, although most authorities agree that levels below 20-30 ng/mL constitute at least a mild deficiency, with severe vitamin D deficiency beginning at a level of 12ng/Ml.9, 10 Szymczak-Pajor et al concluded with the evidence showing that vitamin D deficiency can be a contributing factor in developing both type 1 and type 2 diabetes.11 From the past studies, it was proven that a bidirectional relationship between type II diabetes and periodontal disease. Diabetes influences the periodontium mainly due to the classic micro and macrovascular diabetic complications, changes in subgingival microbiota, and alterations in the host immunoinflammatory response to potential periodontal pathogens.12 Diabetes may result in impairment of neutrophil adherence, chemotaxis, and phagocytosis, which may facilitate bacterial persistence in the periodontal pocket and further resulting in progressive periodontal destruction.13 In a study by Teshome A et al, it was proved that the control of periodontal infection has an impact on the improvement of glycaemic control in diabetic patients. But there is also a significant study that showed that the secretion of insulin is reduced in vitamin D deficiency.14 From a recent study, it was evidenced that supplementation of vitamin D has improved glycaemic control and insulin sensitivity in Type II diabetes patients. Its supplementation also showed a potential anti-inflammatory effect and its active metabolite, 1-25 dihydroxy vitamin D inhibits cytokine production.15 From a study by Joseph et al, it was shown that Vitamin D deficiency results in increased inflammation, bone loss, and through its immunomodulatory effects, it also has a role in periodontal disease progression.16 Since a bidirectional relationship exists between periodontitis and diabetes.17 From the study it can be hypothesized that assessing 25(OH) D serum levels in patients with CGP and Type II diabetes mellitus can help in early diagnosis of bone destruction levels and can be advised with supplementation of Vitamin D along with proper oral and antibiotic prophylaxis, thus decreasing the destruction in bone levels.
Limitations
The size of the groups taken was minimal to compare the biological values of serum.
Vitamin D levels depend on the overall nutritional status of a patient which has to be evaluated with BMI index and another metabolic status of the patients, which was not considered in the present study.
Periodontal disease is a site-specific disease that requires analysis of fluids in the localized site, but evaluating serum levels may trigger some lack of specificity.
Conclusion
Within the limitations of this study, it can be concluded that vitamin D3 levels were low in both the groups but the significant median levels were low for CGP with Type II diabetes mellitus patients. Low levels in healthy patients can be due to a lower intake of dietary calcium coupled with inadequate sun exposure.
