Introduction
Cigarette smoking is associated with bone loss in chronic periodontal disease1, 2 and is considered true risk factor for periodontitis.3 The relative risk of increased periodontal disease increases with heightened exposure to cigarette smoking in a dose-response attachment loss, a higher percentage of tooth mobility and furcation lesions are associated with smoking.4, 5, 6 Periodontal bone loss and tooth loss in smokers can be 12.1% in those aged 20 to 40 years and exceed 55% in those between the ages of 50 and 70 years.7 A recent study in Riyadh, Saudi Arabia, found men who were cigarette smokers to have more missing teeth and a poorer periodontal condition than non-smokers.8 Chronic smoking in the long-term induces an increased rate of alveolar bone height reduction and cessation of smoking can reverse the rate of reduction towards that which is observed in non-smokers.9, 10 Even with a lower usage of tobacco in younger populations, smoking is considered as a potential risk factor for alveolar bone loss.11
Vertical interproximal bone loss is a pattern of bone destruction present in chronic periodontal disease.12 Smoking has a destructive effect on periodontal bone height, including both horizontal and vertical patterns.9 An increased occurrence of vertical bone defects has been observed in cigarette smokers as compared to non-smokers.13 Smoking is considered a potential risk factor for the prevalence and severity of vertical alveolar bone loss,14 whereby the estimated developmental risk for such lesions may be two- to three-fold elevated in current smokers.12 A prospective longitudinal study in Sweden over a period of 10 years, showed a significant increase in the severity of vertical bone loss in smokers, as compared to non-smokers. This study showed that the effects of heavy smoking exposure over a 10-year period can increase the risk for vertical bone loss by five to six times as compared with non-smokers.14 A study in Jeddah, Saudi Arabia, found the estimated risk for bone loss of 30% or more of the root length to be 4.3-fold elevated in cigarette smokers as compared with non-smokers.13
Alveolar vertical defects may be localized within specific segments of the dentition. The majority of such defects have been observed in posterior segments of the dentition.15 Other studies have described more bone loss involving the lingual aspects of maxillary molars and mandibular incisors.4, 16 A Brazilian population study also showed that the group of incisal teeth in smokers were significantly more affected than in non-smokers.5
Radiographic parameters of periodontal status such as marginal bone loss and numbers of missing teeth are higher in cigarette smokers as compared to non-smokers. 8 The usage of digital panoramic radiographs has been proven to be effective in determining the prevalence of intrabony defects in both the mandible and maxilla. 17, 18 It is a rapid and relatively less expensive method, and has a lower radiation dose when compared to a full mouth set of periapical radiographs.17, 19
According to Baljoon et al (2004)12 only a few studies have been done on the association between smoking and the severity of vertical alveolar bone defects. Also, up to date, no such study has been undertaken in the Eastern province of Saudi Arabia. A pilot study was thus undertaken utilizing a convenient sample of patients’ records attending the Dental College Teaching Hospital at the Imam Abdulrahman Bin Faisal University, in the Eastern Province of Saudi Arabia.
Materials and Methods
A cross-sectional retrospective study on a convenient sample of 60 orthopantomographic records of patients with chronic periodontal disease was done. Records were conveniently selected of patients attending the Dental College Teaching Hospital at Department of Periodontics, Chandra Dental College & Hospital, Safedabad, Barabanki. Data was collected from patients’ charts from the previous 2 years. Of the above 60 patients, 30 were smokers with a history of smoking of more than 2 years, including having smoked > 10 cigarettes per day,20 and 30 were non-smokers with a history of never had smoked before. Patients had to be older than 21 years, so as to avoid the effects of bone development, and under 50 years old to avoid the effects of menopause.
Other exclusion criteria for patients, which were confirmed from patients’ records, were the following: lactating and/or pregnant females, history of bone metabolism disease, active osteoporosis treatment, history of radiotherapy, diabetes mellitus, AIDS, epilepsy, cardiovascular, renal and hepatic disorders, crowded teeth, occlusal trauma, tobacco chewers, usage of antibiotics, non-steroidal anti-inflammatory drugs and/or steroids within the past 6 months, and patients who have undergone periodontal treatment within the past 6 months.
Three blinded examiners performed the measurements with reference to the smoking status of the patient. These patients were afterwards separated into smoking and non-smoking groups.
Patients had to have at least 20 teeth present, and the radiographic data was obtained from patients’ digital records utilizing extraoral digital panoramic radiographs (Gendex Orthoralix 9200 DDE, Georgia-USA). Bone height measurements were made from the radiographs using the MiPACS Dental Enterprise Viewer Program (Medicor Imaging, Charlotte-USA).
To determine the severity of vertical alveolar bone loss, the vertical distance from 2mm below the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured on the proximal surfaces of all teeth.8 The most coronal point of the alveolar bone adjacent to the proximal surface including a periodontal ligament space showing a normal radiographic width, was con- sidered as the ABC.21 The height of the interproximal alveolar bone was measured mesially and/or distally, as applicable, to each tooth surface. All teeth were assessed, except third molars. In cases where either the dental or bony landmarks could not be identified on either mesial or distal aspects, the tooth was then excluded from the study. A tooth was also considered as non-measurable if the CEJ or the ABC could not be properly identified due to overlapping, caries or restorations.
Inter-examiner reliability with respect to vertical bone defect measurements was estimated using 10% of the total sample size, utilizing the weighted Co- hen’s kappa ĸ statistic. The inter-examiner reliability was ĸ = 0.83. The correlation coefficient was r = 0.998 (p<0.001). It was concluded that errors related to the variability of inter-examiner assessments did not affect the outcome. Intra-observer reproducibility to test error of measurement regarding the precision of the bone height measurement procedure was performed for each of the three investigators by means of replicating measurements from 5% of the total sample size at the beginning and at the end of the assessment period. The reproducibility with regards to the mean bone height per patient was expressed as the precision (s), i.e. the standard deviation of a single measurement. The estimates of precision for the three investigators were s1 = 1.04, s2 = 0.87 and s3 = 0.87 respectively. The influence on the observer variation of mean vertical bone defects was therefore regarded as being minimal.
Statistical analysis was performed using SPSS-20.0 (IBM product, Chicago-USA). Numerical data were explored for normality by checking the distribution of data and using tests of normality (Kolmogorov-Smirnov test). The tests revealed a normal distribution, presented as Mean ± Standard Deviation. The unpaired t-test was applied to compare the mean vertical alveolar bone loss between smokers versus non-smokers. The non-parametric Wilcoxon Mann-Whitney U test was applied to compare the mean vertical alveolar bone loss in relation to groups of teeth between smokers and non-smokers. The Wilcoxon Mann Whitney U test for non-Gaussian unbalance group distribution for comparison of genders at 5% level of significance was applied to determine gender-wise differences of mean millimeter vertical bone loss in groups of teeth between smokers and non-smokers. A p-value ≤0.05 was considered to be a statistically significant result.
Results
Among 60 patients, there were 30 smokers and 30non-smokers. Of these 31(51.7%) were males and 29(48.3%) were females, with almost a 1:1 male to female ratio. The mean age was 41.85±7.38 (ranging from 23 to 50) years. A total of 3 patients from both smoker and non-smoker groups presented with no vertical alveolar bone loss (Table 1). From a potential total of 2280 surfaces, those tooth surfaces which included the no nidentification of either bony or dental landmarks, as well as the CEJ or ABC, were not included. Therefore, a total of 565 surfaces were studied which presented with vertical alveolar bone loss. This comprised 302 surfaces in smokers and 263 surfaces in non-smokers. Males presented with 306 surfaces and females with 259 surfaces. 269 were male smokers and 33 were female smokers, and 37 were male non-smokers while 226 were female non-smokers. (Table 1)The average prevalence of surfaces with vertical alveolar bone loss in the smoker group of patients was 10.06%, and 8.76% in the non-smoker group. (Table 1) The overall mean millimeter vertical bone loss for a total of 60 patients was 1.023±0.835 mm (Table 1). There was a greater mean millimeter vertical bone loss in smokers as compared to non-smokers (1.112±0.784 mm vs. 0.935±0.887 mm respectively), however this difference of means was not statistically significant (p=0.418) at a 5% level of significance (Table 1).Table 2 depicts the severity of vertical alveolar bone loss in different groups of teeth in smokers as compared to non-smokers. There was a significantly higher mean millimeter vertical bone loss in the pre- molar group of teeth in smoker patients as compared to non-smokers (1.416±1.234 mm; p=0.030) (Figure 1). When the surfaces of affected teeth were separated into maxillary and mandibular groups of teeth, there was a statistically significant difference (p=0.002) between smokers and non-smokers, depicting a greater mean millimeter vertical bone loss in specifically the maxillary premolar group of teeth (1.619±1.816 mm) (Table 3). Maxillary molar groups of teeth were also more affected in smokers as compared to non-smokers, although this was not statistically significant (p=0.740) (Table 3). Furthermore, any gender-wise differences of mean millimeter vertical bone loss between the different groups of teeth in smokers as compared to non-smokers were found to be non-significant (Table 4).
Discussion
Figure 1
Differencesof mean millimeter vertical bone loss in groups of teeth between smokers and non-smokers. (P-value = 0.030)
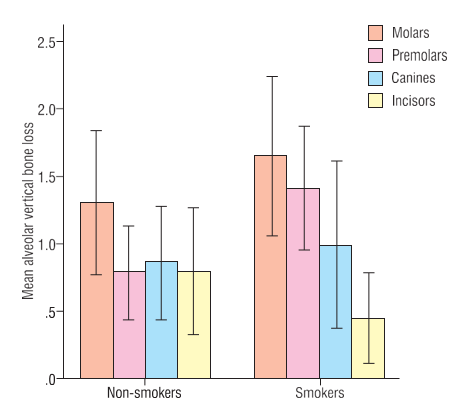
Table 1
Distribution of smokers and non-smokers with gender and surfaces and mean millimeter vertical bone loss
Table 2
Differences of mean millimeter vertical bone loss in groups of teeth between smokers and non-smokers
Table 3
Differences of mean millimeter vertical bone loss in maxillary and mandibular groups of teeth between smokers and non-smokers
Table 4
Gender-wise differences of mean millimeter vertical bone loss in groups of teeth between smokers and non-smokers
All patients in this study exhibiting intra-alveolar bone defects had been diagnosed with chronic periodontitis, and any vertical alveolar bone loss was due to the accumulated effects since the initiation of the disease.22 The effects of bone development and menopause were excluded. Other local and systemic factors which may have influenced the pathogenesis of periodontal dis- ease were also excluded, including any periodontal or antibiotic therapy within the previous 6 months.
Digital panoramic radiographs were used to determine the prevalence of intrabony defects in both the mandible and maxilla. The radiographic method applied in this study to evaluate marginal bone loss was similar to that of a previous study.8 The height of the periodontal bone on the proximal surfaces of teeth were determined as the vertical distance from 2mm below the CEJ to the ABC, whereby 2mm was considered as the distance found in normality.8 This method was also proposed in other studies, except 1mm was subtracted in those studies.5, 23 A study using open surgery measurement as the gold standard showed that an underestimation of bone loss can range from 13% to 32% for panoramic films. Furthermore, they do not provide information on the height of the vestibular or lingual periodontal bone.24
A relatively new imaging modality, namely cone- beam computed tomography (CBCT) which is dedicated to dentomaxillofacial radiology, has been introduced for applications in periodontology. Obvious advantages such as relatively low-cost and low-dose have been reported when compared to digital panoramic radio- graphs.25 CBCT images also demonstrate more potential in the morphological description of periodontal bone defects, however, digital radiography provides more bone details.26 Furthermore, CBCT does not offer a significant advantage over conventional radiography for assessing periodontal bone levels, bone quality and periodontal ligament space, whereas bony defects, craters, and furcation involvements seem to be better depicted on CBCT.25 Future studies utilizing CBCT in the field of periodontology are certainly indicated, however, consideration of its advantages, limitations, and risks should be taken into account.25
In this study the severity of vertical radiographic bone loss was greater in smokers as compared to non-smokers, although this finding was not significant. Furthermore, the average prevalence of surfaces with vertical alveolar bone loss in the smoker group of patients was greater than in the non-smoker group. This study thus confirms previous cross-sectional13, 5, 10, 12 and longitudinal studies9, 14, 27 showing an association between smoking and the prevalence and severity of vertical alveolar bone loss, signifying smoking to be a potential risk factor. A limitation of this study however is the absence of discernment between light and heavy smoking exposure. A dose-response relationship between severity of vertical bone loss and heavy smoking exposure has been demonstrated to strengthen the probability of smoking as a significant risk factor.28, 13, 29 Studies have confirmed greater alveolar bone destruction, including increased clinical attachment loss and tooth mobility among heavy smokers.22
A significant difference in mean vertical bone loss in premolar groups of teeth between smokers and non-smokers was found in this study. Specifically, this was found to be significantly associated with maxillary premolars. Similar findings in other studies of smoker patients have observed such bone defects in the posterior segments of the dentition, specifically the maxillary molars.15, 16 In this study maxillary molar groups of teeth were also more affected in smokers as compared to non-smokers, although this finding was not significant. The findings in this study are however in contrast to other studies which found incisor teeth to be more affected.4, 5 Other studies have however found no increase in the deleterious effects of smoking in specific areas of the dentition.30 Furthermore in this study, no significant differences were found between males and females regarding specific groups of teeth being affected in smokers as compared to non-smokers. More longitudinal studies are needed to better elucidate the effects of smoking on particular groups of teeth,5 including any differences regarding gender.
In this study gender differences were not found to be significantly associated with the prevalence or severity of vertical bone loss between smokers and non-smokers. Meta‐analysis of population surveys has however shown males to be at greater risk for destructive periodontal disease than females; this being due to differential gene regulation of sex steroid-responsive genes.31 Other studies however have indicated no significant effect of gender on periodontal health and have attributed smoking, poorer oral hygiene and a less positive attitude towards oral health, rather than any genetic factor, to increase susceptibility to periodontal disease among males.13, 32, 33 Furthermore, the prevalence of smoking has been found to be much higher in males compared to females in various population studies.8, 34, 35 However, as a limitation of this study, any associations between clinical indicators of periodontal disease, oral hygiene practices and smoking with regards to gender differences were not studied. Further studies are needed regarding the association of vertical radiographic alveolar bone loss with smoking, gender, and periodontal status.8
In the present cross-sectional study, convenience sampling was utilized. A limitation of this study is that the sample size (60 subjects) may not be representative of the population as a whole36 However, the present study was a pilot study performed on a local population attending the Dental College in the Eastern province of Saudi Arabia, and comparisons were made with other population groups. However, the epidemiology of periodontitis can be influenced by various factors within various geographical populations showing different demographic and ecological characteristics.37 Also, the selection of criteria specific to the definition and magnitude of the prevalence of periodontal disease can differ between studies, making comparisons difficult with data from other populations, both in developing and developed countries.5, 38 Underprivileged socio-economic status and poor educational status are also known significant risk factors of periodontal disease and marginal bone loss.39 More studies on prevalence incorporating broader demographic parameters within different regions of Saudi Arabia should be envisaged, thereby enabling more meaningful comparisons to be made.
The effect of smoking on the progression of periodontal disease which includes significantly influencing alveolar bone loss has been well proven.40 Nicotine, which is cytotoxic, and its metabolites may have both local and systemic effects, although nicotine absorption from smoke in the oral cavity has been indicated to be low.14, 41 The local effects of nicotine involve acting directly as an irritant on the gingiva and alveolar bone.42 Systemic effects may cause alterations in the cellular immune response, including an increase in proinflammatory cytokine secretion, thereby affecting bone turnover.43, 44 Other systemic effects may include a resultant insufficient vascular supply, as well as having detrimental effects on bone cells, leading to alveolar bone loss.45 Nicotine can significantly increase the levels of prostaglandin and interleukin 1-beta, which are inflammatory mediators of osteoclastic bone resorption.46 Nicotine has also been suggested to stimulate bone matrix turnover, including an increase in matrix metalloproteinases, thereby favoring bone matrix resorption.47 Other studies have described a potential change in the receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin (OPG) ratio. A systemic nicotine-induced suppression of OPG production can thus lead to bone loss in smokers.[49.50] Long-term observational studies have shown that exposure to cigarette smoke elevates the probability of severe vertical bone loss in chronic smokers.14 This study has similarly shown an increased probability of the detrimental effects of tobacco smoking on specifically vertical alveolar bone loss. Smoking per se may not however be the only cause, as other factors may also play a role.14 A limitation of this study was to not include these factors, although mention shouldbe made of their relevant importance. Clinical studies have shown a strong correlation between attachment loss and the number of smoking years, whereby severe periodontal disease can be found in 45% of those who smoked for more than 10 years.48 The long-term (10 years) influence of smoking on periodontal bone height has been demonstrated to cause a reduction in bone height 2.7 times greater in adult smokers.49 Smoking cessation can however lead to an improvement in periodontal parameters, regardless of the duration of smoking.46
The presence of plaque as well as the rate of plaque formation is not significantly related to severity of vertical bone defects in smokers.12, 46, 49 This has been ascribed to motivated smokers exercising plaque control as efficiently as non-smokers. Increased calculus de- posits associated with smoking are also not considered as having a major influence on periodontal health.46
Scarce and conflicting reports question whether smoking has an effect on oral microbiota.50 Various oral surfaces display different receptors for bacterial adhesion whereby microbial niches can be resilient to disturbances in the surrounding environment induced by smoking.50 Nicotine and its metabolite cotinine, which are increased in crevicular fluid of smokers,51 can increase the adhesion of specific bacteria to epithelial cells in periodontal pockets.52 It has been suggested that differences in the prevalence of microbiota are possibly related to differences in pocket depth between smokers and non-smokers, and not due to smoking having an influence on the composition of subgingival microflora, but rather contributing to periodontal destruction by inducing changes within the periodontium.13 In contrast, a study described an alteration of the microbial community in smoker patients, whereby smoking may aggravate the subgingival dysbiosis that characterizes chronic periodontal disease.53 Other studies have also described smokers with periodontal disease to have a distinct and less diverse microbial community, including inducing the depletion of beneficial bacteria and increasing periodontopathic bacteria.4, 54, 55 However, the issue whether smoking selectively alters the subgingival microbiota or causes changes in the host response leading to deeper periodontal pockets, thereby favoring the colonization of specific periodontal pathogens, remains controversial.56 Controversy also exists about the effect of gender on the presence of subgingival bacteria. Studies have reported a difference in the bacterial profile between males and females,57, 58 while other studies have indicated gender to have no effect on the prevalence of subgingival bacteria in periodontitis patients, as well as in healthy controls.59, 60
Conclusions
Within the limitations of this study, the role of smoking is supported to be considered a risk factor for the development of vertical alveolar bone defects. Smokers presented with more severe vertical radio- graphic alveolar bone loss as compared to non-smokers, whereby maxillary premolars were significantly more affected. No significant differences were found between males and females regarding specific groups of teeth being affected in smokers as compared to non-smokers. Further studies incorporating broader demographic data are needed to examine the prevalence and severity of vertical radiographic alveolar bone loss in smokers, including the effects on specific groups of teeth, as well as the association with gender. Future studies are suggested including comparisons between various radiographic techniques (FMX, OPG, CBCT) in the detection of alveolar bone loss, this being in relation to periodontal diseases among smokers and non-smokers. Vertical bone loss is associated with further periodontal bone loss and tooth loss; thus, the clinical importance of the early detection thereof is imperative. Emphasis on education of patients and the utilization of smoking cessation programs can bring about positive results in patients suffering from periodontal disease.
