- Visibility 162 Views
- Downloads 43 Downloads
- Permissions
- DOI 10.18231/j.ijpi.2024.030
-
CrossMark
- Citation
Specialized pro-resolving mediators - Key players in resolution
Abstract
Inflammation is an essential biologic response observed across species with particular importance to human health and disease. The primary objective of the inflammatory response is to eliminate the initial cause of inflammation and restore tissue homeostasis. Effective resolution of inflammation is essential for maintaining health; this process is active and marked by a shift from the production of classic lipid mediators like prostaglandins and leukotrienes to the synthesis of specialized pro-resolving mediators (SPMs). These include arachidonic acid-derived lipoxins, aspirin-triggered lipoxins, eicosapentaenoic acid-derived resolvins of the E-series, docosahexaenoic acid-derived resolvins of the D-series, as well as protectins and maresins. Understanding the biosynthesis, mechanisms of action, and therapeutic potential of SPMs is crucial for developing strategies to manage inflammatory diseases and improve health outcomes. This article reviews the current knowledge of SPMs, and their roles in inflammation resolution.
Introduction
Inflammation is an essential mechanism in human health and diseases. Roman scientist Cornelius Celsus in the 1st century defined the clinical signs of inflammation. Initially, four cardinal signs of inflammation were identified; rubor et tumor cum calore et dolore (redness, swelling with heat and pain) in 1858. In 1958, Virchow on cellular basis of inflammation as a pathologic condition, added a new cardinal sign, functio laesa (loss of origin/tissue function). [1], [2]
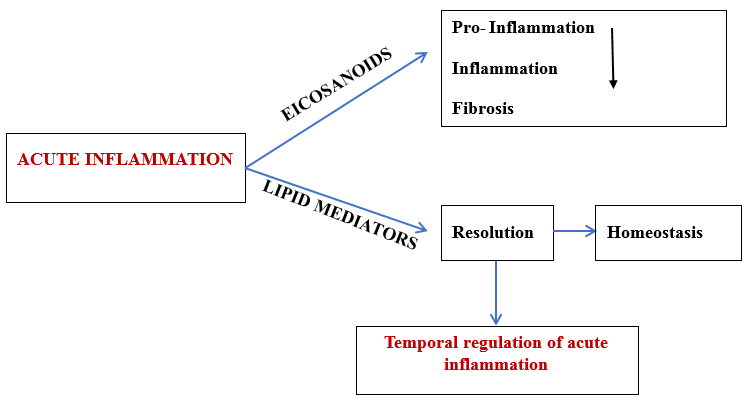
The primary goal of the inflammatory response is to detect and eliminate functions that interfere with homeostasis. A typical inflammatory response consists of 4 components. Inflammatory inducers, detecting sensors, downstream mediators, and the target tissues. [3], [4] Resolution of inflammation is an active process and this process is normally maintained by endogenous mediators – Specialized pro-resolving mediators.(SPMs). [5], [6], [7] ([Figure 1])
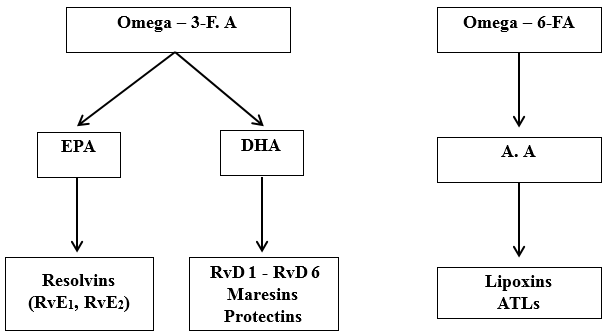
These are a group of fatty acids, an essential part of the cell membrane and act as metabolic fluid for mammalian tissue. They also function as signalling molecules. Omega -6 fatty acid (Linoleic acid-LA) and omega -3 fatty acid (Linolenic acid-ALA) are essential fatty acids that cannot be biosynthesized by humans/ other animals. LA and ALA undergo sequential desaturation reaction and transform into their higher form which are in their unsaturated form. Arachidonic acid (A.A) is formed from LA and EPA (eicosapentaenoic acid), and DHA (docosahexaenoic acid) from ALA. [8], [9]
The pro-resolving lipid mediators include Lipoxins, Protectins, Resolvins and Maresins. Lipoxins (LXs) and aspirin triggered lipoxins (ATLs) are formed from arachidonic acid which mainly decrease inflammation and assist resolution. Omega-3 FA is the source of resolvins, maresins and protectins. [8] ([Figure 2])
Discussion
Lipoxins
They are natural pro-resolving mediators derived from endogenous F.A. They have strong dual actions, i.e. anti-inflammatory and resolution actions. The first identified lipoxins are lipoxin A4 and B4. [10], [11]
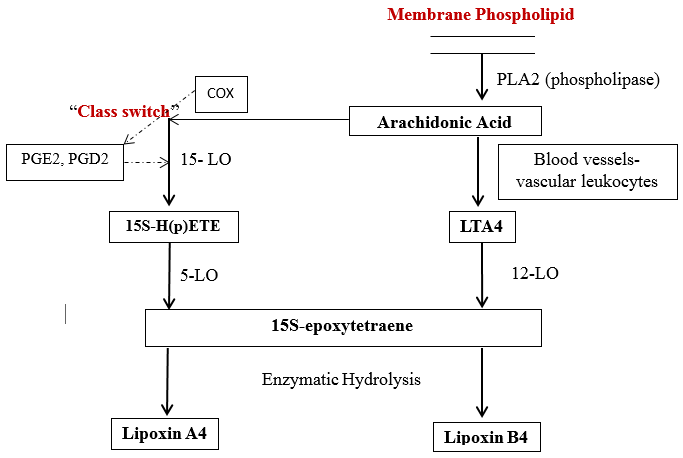
Biosynthesis of lipoxin- 3 main pathways has been identified. [12]
In blood vessels- Human platelets do not have the ability to produce lipoxins on their own, but become the major source of lipoxin, when they adhere to PMNs (polymorphonuclear neutrophils), i.e. the formation of lipoxin is initiated by platelet-leukocyte interactions. Initially, there is transcellular conversion of 5-LO (lipoxygenases-LOs) product, LTA4(leukotriene A4). When platelet get adhere, 12-LO converts this LTA4 into lipoxin A4 and B4(LXA4 and LXB4 – positional isomers).
Classical pathway- In human mucosal tissue, GI tract, oral cavity, the sequential oxygenation of A.A by 15-LO and 5-LO followed by enzymatic hydrolysis leads to the production of lipoxin A4 and B4, i.e. when epithelial cells get activated, they generate and release 15S-HETE (hydroxy-eicosatetraenoic acids), that is taken up by the PMN and converted to lipoxin with help of 5-LO.[Figure 3])Ecosanoid class switch – during inflammation, when there is PMN infiltration, there is coordinated coincidence of LT and PMN, which is associated with spontaneous resolution of the condition. When human blood PMN is exposed to PGE2(prostaglandins), which triggers eicosanoid class switch by regulating 15-LO. These event block LTs(leukotrienes) formation, thereby regulates leukocytes, PGD2 and HETE .[12] ([Figure 3])
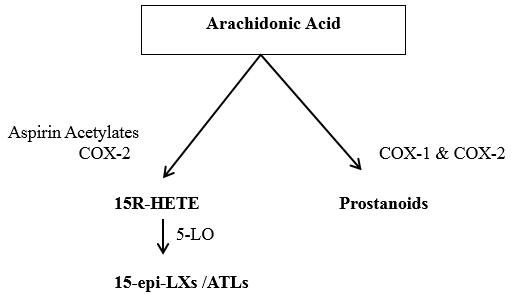
The synthetic pathway by aspirin is mainly seen in cells that bear COX-2 like vascular endothelial cells, epithelial cells, monocytes and macrophages. When aspirin is administered during inflammation, the stimuli induce COX-2 to generate 15R-HETE, this is rapidly converted to 15-epimeric-LX / aspirin triggered by lipoxins by 5-LO. [13], [14], [15] ([Figure 4])
Cellular functions of lipoxins
Decreases adherence of leukocytes
Reduces vascular leakage.
Lowers prostaglandin E2 levels in exudates.
Reduces the number of apoptotic neutrophils.
Inhibit neutrophil recruitments.
Attenuates expression of the nuclear factor kappa B gene.
Blocks leukocyte adhesion protein-1 and chemotaxis
Promotes lymphatic removal of phagocytes
Inhibits T- cell adhesion to vascular and salivary epithelium.
Enhances microbial phagocytic function. [16]
Resolvins
These are endogenous lipid mediators produced during the resolution phase of acute inflammation from essential fatty acid (EPA – eicosapentaenoic acid & DHA- docosahexaenoic acid). Two series of resolvins are there, E series from EPA and D series from DHA. [17], [18]
Biosynthesis of resolvins
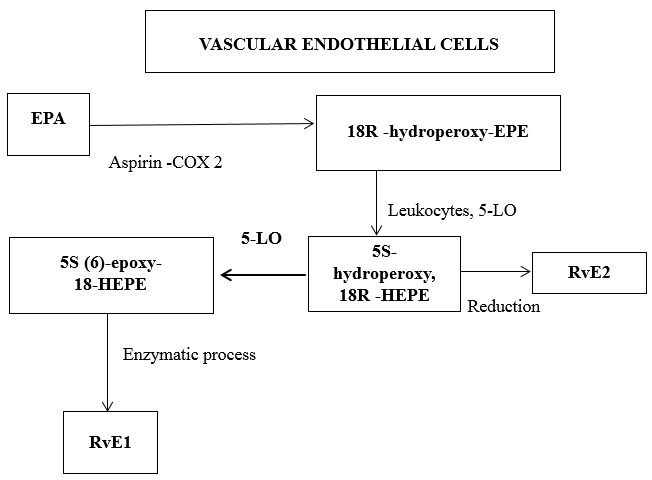
The first bioactive molecule identified among resolvins is RvE1- Resolvin E1. Vascular endothelial cells initiate the biosynthesis through 2 pathways.
Aspirin modified COX-2 coverts EPA into 18R-hydroperoxy-eicosapentaenoic acid (18R-HPEPE) and 18S-hydroperoxy-eicosapentaenoic acid (18S-HPEPE). Aspirin impacts the formation of Resolvin E1 by acetylating COX-2 in vascular endothelial cells, that stereo selectively generate 18R-HPEPE. This is taken up by human monocytes and metabolized to RvE1 and RvE2 by leukocytes 5-LO. ([Figure 5])
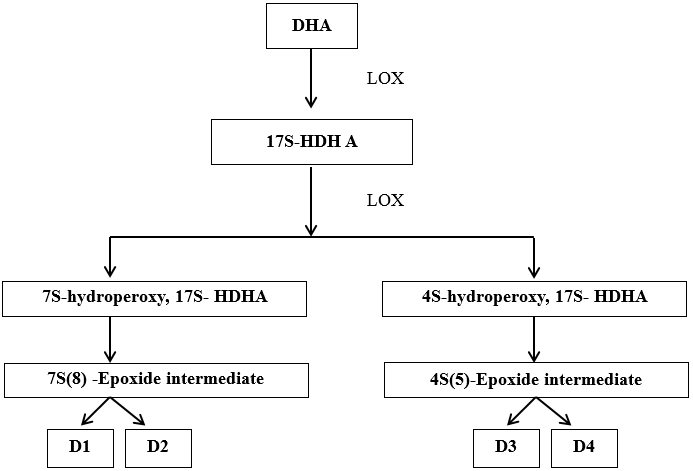
Resolvin D is formed from DHA. The LOX product 17S-hydroxy-DHA (17S-HDHA) which is rapidly transformed by the LOX activity in human PMNs into 2 epoxides intermediate. This open to form a bioactive product – 17S resolving series (RvD series). [19], [20] ([Figure 6])
Functions of resolvin E1 [6], [17], [21]
Inhibits neutrophil infiltration
Modulates chemokine/cytokine synthesis
Promotes healing of inflamed tissues and bone regeneration
Enhances phagocytosis
Activates lymphatic removal of phagocytes
Attenuates systemic production of c- reactive protein and interleukin-1
Reduces eosinophil and lymphocyte recruitment
Regulates adipokines.
Decreases inflammatory actions of COX-2
Attenuates expression of the nuclear factor kappa-B gene
Reduces the monocytes concentration
Increases CD55 expression on epithelial cell and PMN cell clearance
Rescues impaired phagocytosis in LAP patient macrophages
Prevents rejection of allografts
Activates anti-apoptotic signals.
Functions of resolvin D1 [22], [23]
Inhibits neutrophil recruitment
Anti-hyperalgesic properties
Shortens resolution interval
Reduces oxidative stress-mediated inflammation
Attenuates agonist pain molecules
Induces macrophage phagocytosis
Stimulates M2 macrophage phenotype
Temporally regulates micro RNAs
Reduces cytokines in broncho alveolar lavage fluid
Ameliorates insulin sensitivity
Enhances microbial clearance
Reduces level of prostaglandins and leukotrienes.
Protectins
Endogenous lipid mediators, also called as neuroprotectins, account for the protective actions observed in neural tissues and within the immune system.
Biosynthesis
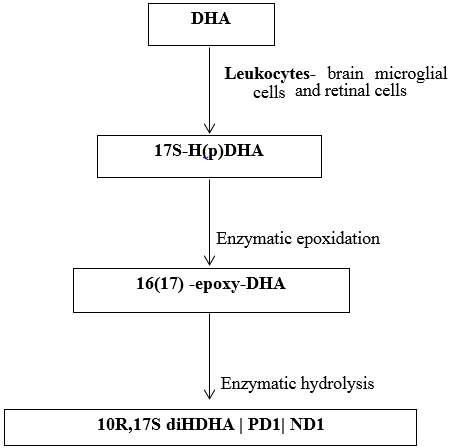
Endogenously produced DHA is converted into triene containing conjugated structure via LOX pathway defines the key feature of the family derived from DHA. LOX product 17S-H(p)DHA undergoes enzymatic epoxidation to 16(17)-epoxide, that is enzymatically converted to 10,17 dihydroxy containing bioactive product- 10R,17S diDHA, NPD1/PD1 – protectins/ neuroprotection. [24], [25] ([Figure 7])
Human peripheral blood lymphocytes also have the ability to produce protectin D1 with a Th2 phenotype there by reducing TNF-α and interferon γ secretion, and thereby blocking T cell migration and promoting T cell apoptosis. [26]
Functions [17], [27]
Decreases inflammatory actions of COX-2
Inhibits neutrophil infiltration
Modulates chemokine/cytokine synthesis
Regulates T-cell migration
Regulates macrophage function
Upregulates CCRS expression on apoptotic leukocytes
Prevents hepatocyte steatosis
Inhibits pain signals
Suppresses eosinophil chemotaxis and adhesion.
Maresins
They are macrophage mediators in resolution of inflammation, primary molecules produced by macrophages with homeostatic function. They are the third largest family of SPMs derived from DHA 20. [6], [28]
Biosynthesis
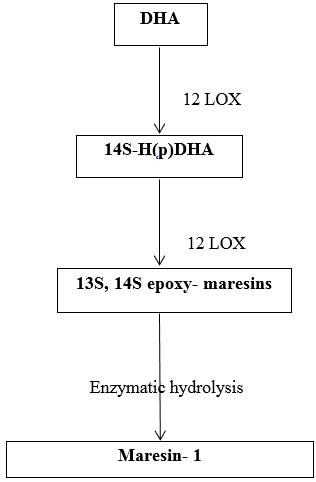
It occurs mainly in M2 macrophages and is initiated by human macrophage 12- lipoxygenase (12-LO).
Initially, DHA is converted enzymatically into14-hydroxyDHA intermediate. This requires the addition of oxygen atom into DHA at the 14th carbon atom. This give rise to 14S- H(p)DHA, which is further metabolized to 13S, 14S- epoxide maresin, again undergoes enzymatic hydrolysis to form maresin-1. [20] ([Figure 8])
Functions [29]
Reduces neutrophil number in exudate
Enhances macrophage phagocytic functions
Decreases trans endothelial polymorphonuclear cell migration
Conclusion
Acute inflammation serves to protect the host and is primarily initiated by neutrophils in response to a challenge. The outcome of inflammation depends on a balance between factors that either amplify the inflammatory response or promote its resolution. Recent insights reveal that the resolution of inflammation is regulated by protective mediators such as lipoxins derived from arachidonic acid, aspirin-triggered lipoxins, E-series resolvins derived from eicosapentaenoic acid, D-series resolvins derived from docosahexaenoic acid, protectins, and maresins.
These lipid mediators interact with G protein-coupled receptors on innate immune cells to facilitate several processes: they halt leukocyte infiltration, restore normal vascular permeability and reduce edema, promote the apoptosis of polymorphonuclear neutrophils, encourage the non-inflammatory infiltration of monocytes/macrophages, and assist macrophages in clearing apoptotic neutrophils, bacteria, and necrotic debris from the affected area. Through these processes, inflammation resolves effectively, leading to a return to homeostasis.
Source of Funding
None.
Conflicts of Interest
None.
References
- Virchow R. . Cellular pathology, as based upon physiological and pathological histology : twenty lectures delivered in the Pathological Institute of Berlin during the months of February, March and April, 1858. 1858. [Google Scholar]
- Freire M, Dyke TV. Natural resolution of inflammation. Periodontol 2000. 2000;63(1):149-64. [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771-6. [Google Scholar]
- Fierro IM, Serhan CN. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz J Med Biol Res. 2001;34(5):555-66. [Google Scholar]
- Freire M, Dyke TV. Natural resolution of inflammation. Periodontol. 2000;63(1):149-64. [Google Scholar]
- Serhan C, Clish C, Brannon J, Colgan S, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2- nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197-204. [Google Scholar]
- Dyke TV. Inflammation and periodontal diseases: a reappraisal. J Periodontol. 2008;79(8):1501-2. [Google Scholar]
- Dyke TV, Serhan C. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82(2):82-90. [Google Scholar]
- Serhan C. A search for endogenous mechanisms of anti_inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122(4):305-21. [Google Scholar]
- Filep J, Zouki C, Petasis N, Hachicha M, Serhan C. Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94(12):4132-42. [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis N, Hogg N, Brady H. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164(4):1663-7. [Google Scholar]
- Serhan C, Chiang N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entrée for resoleomics. Rheum Dis Clin North Am. 2004;30(1):69-95. [Google Scholar]
- Levy B, Clish C, Schmidt B, Gronert K, Serhan C. Lipid mediator class switching during acute inflammation: sig_nals in resolution. Nat Immunol. 2001;2(7):612-9. [Google Scholar]
- Serhan C. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68-69:433-55. [Google Scholar] [Crossref]
- Gewirtz A, Fokin V, Petasis N, Serhan C, Madara J. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am J Physiol. 1999;276(4):988-94. [Google Scholar]
- Serhan C, Chiang N, Dalli J, Levy B. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb Perspect Biol. 2014;7(2). [Google Scholar] [Crossref]
- Bannenberg G, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger K. Molecular circuits of resolution: formation and actions of resolvins and protec_tins. J Immunol. 2005;174(7):4345-55. [Google Scholar]
- Yang R, Chiang N, Oh S, Serhan C. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011;14. [Google Scholar] [Crossref]
- Serhan C, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(1):200-15. [Google Scholar]
- Serhan C. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolv_ing mediators. J Thromb Haemost. 2009;7(1):44-8. [Google Scholar]
- Dyke TV. Cellular and molecular susceptibility determinants for periodontitis. Periodontol 2000. 2000;45:10-3. [Google Scholar] [Crossref]
- Hong S, Gronert K, Devchand P, Moussignac R, Serhan C. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J Biol Chem. 2003;278(17):14677-87. [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh S, Vickery T, Schmidt B. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484(7395):524-8. [Google Scholar]
- Bannenberg G, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger K. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins1. J Immunol. 2005;174(7):4345-55. [Google Scholar]
- Chen P, Fenet B, Michaud S. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583(21):3478-84. [Google Scholar]
- Marcheselli V, Mukherjee P, Arita M, Hong S, Antony R, Sheets K. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82(1):27-34. [Google Scholar]
- Schwab J, Chiang N, Arita M, Serhan C. Resolvin E1 and protectin D1 activate inflammation-resolution pro_grammes. Nature. 2007;447(7146):869-74. [Google Scholar]
- Gireddy H, Rajaram H, Koduganti R, Ambati M, RA, Harika T. Maresins: The Mainstay in Periodontal Resolution. Cureus. 2022;14(1). [Google Scholar] [Crossref]
- Shinohara M, Mirakaj V, Serhan CN. Functional metabolo_mics reveals novel active products in the DHA metabolo_me. Front Immunol. 2012;3. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Joy B, Ramesh R, Nafeesa RB, Jayasree A, Reghunath S, Mathamkuth AE. Specialized pro-resolving mediators - Key players in resolution [Internet]. IP Int J Periodontol Implantol. 2024 [cited 2025 Sep 14];9(3):146-150. Available from: https://doi.org/10.18231/j.ijpi.2024.030
APA
Joy, B., Ramesh, R., Nafeesa, R. B., Jayasree, A., Reghunath, S., Mathamkuth, A. E. (2024). Specialized pro-resolving mediators - Key players in resolution. IP Int J Periodontol Implantol, 9(3), 146-150. https://doi.org/10.18231/j.ijpi.2024.030
MLA
Joy, Bilha, Ramesh, Roshni, Nafeesa, Raseena Beevi, Jayasree, Aswathy, Reghunath, Shruthi, Mathamkuth, Abdurasheed Edakkot. "Specialized pro-resolving mediators - Key players in resolution." IP Int J Periodontol Implantol, vol. 9, no. 3, 2024, pp. 146-150. https://doi.org/10.18231/j.ijpi.2024.030
Chicago
Joy, B., Ramesh, R., Nafeesa, R. B., Jayasree, A., Reghunath, S., Mathamkuth, A. E.. "Specialized pro-resolving mediators - Key players in resolution." IP Int J Periodontol Implantol 9, no. 3 (2024): 146-150. https://doi.org/10.18231/j.ijpi.2024.030
